The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted the public about the sale of mislabelled Chlorpheniramine and Dexamethasone injection ampoules in Nigeria.
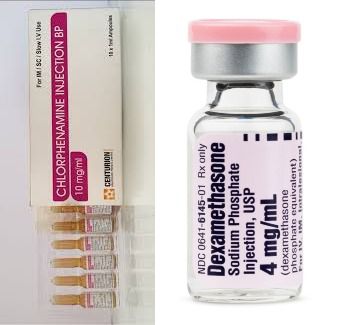
In a public alert (No. 06/2025) made available on the agency’s website, NAFDAC revealed that these injections were repacked and mislabelled as Noristerat Injection 200mg.
It stated that samples of the counterfeit product had been found in Niger State, Bauchi State, and especially at the Coordinated Wholesale Centre (CWC) in Kano State.
NAFDAC also stated that the counterfeiters had been tracked and arrested for further investigation and regulatory enforcement.
The agency clarified that genuine Noristerat 200mg was a contraceptive injection that provided eight weeks of pregnancy protection and was a short-term birth control method.
NAFDAC directed all zonal directors and state coordinators to monitor and remove the falsified products from circulation within their zones and states.
The agency urged importers, distributors, retailers, healthcare professionals, and caregivers to be vigilant in the supply chain to prevent the entry of counterfeit products.
The agency emphasized that medical products should only be obtained from authorized or licensed suppliers and that their authenticity and physical condition should be carefully checked.
NAFDAC encouraged healthcare professionals and consumers to report any suspicions of substandard or falsified medicines or medical devices to the nearest NAFDAC office, through the NAFDAC helpline at 0800-162-3322, or via email at sf.alert@nafdac.gov.ng.
It also urged healthcare professionals and patients to report any adverse events or side effects related to using medicinal products or devices through the nearest NAFDAC office.
REPORTS!
It said the public could also report adverse drug effects using the E-reporting platforms available on the NAFDAC website (www.nafdac.gov.ng) or through the Med-Safety app, available on Android and iOS stores, or via email at pharmacovigilance@nafdac.gov.ng.
NAFDAC said the falsified injection alert would also be uploaded to the World Health Organisation (WHO) Global Surveillance and Monitoring System.























